good afternoon guys , can you please help me to find an easy way to factories?

Thanks I have received the R150 🤩 🙏
Good Day everyone
All thanks to Omelela who assisted me on Empirical formular . 🤗 🙏
Hi guys , on Friday am writing physics , can you help me on how to calculate empirical formular?
Omelela Dlova noted.
Omelela Dlova HOW DO YOU DETERMINE EMPIRICAL FORMULA?-: you will be given couple of elements in grams or in percentages on your exams e.g Na(26.6%/26.6g) , S(34.4%/34.4g) ,O(37.2%/37.2g) .
-:From there you need convert those grams / percentages into number moles using this formula (n=m divided by M )whereby n-is the number of moles , m-is your mass whether given in grams or in percentages , M-is the molar mass which you will find on your Periodic table.e.g (Na : 26.6%. )
n=m/M
n=26.6/23
n=1.16mols
(S : 34.4%. )
n=m/M
n=34.4/32.1
n=1.07mols
(O:37.2%)
n= m/M
n=37.2/16
n=2.33mols
-: From here you need to take the smallest number of moles and divide all of them by that number** in this case the smallest number of moles is 1.07 mols for Sulphur (S) , now divide all the number of moles by the smallest of all**e.g n(Na)=1.16/1.07
=1.1
n=1
n(S) = 1.07/1.07
= 1
n(O)=2.33/1.07
=2.2
=2
Therefore your Empirical formula is Na1S1O2, but you should not write that 1 in the Empirical formula only write numbers which are bigger than 1 then you will therefore have something like this NaSO2 as your final answer.
NOTE : if you got 1 as your answer on every element then your answer will be like this NaSO.
Anathi Vato Thank you @Omelela

Omelela Dlova use a quadratic formula you'll never go wrong.-: for instance if you are given an equation like this : 2x²-x+ 6in the formula below you have a,b and c ,in the above equation your a=2 ,b=-1(since the coefficient of x isn't written automatically you know it's 1) ,c=6 .so in the place of any alphabet in the formula you substitute its value and let the calculator do the rest.I'll also provide done examples please look into them.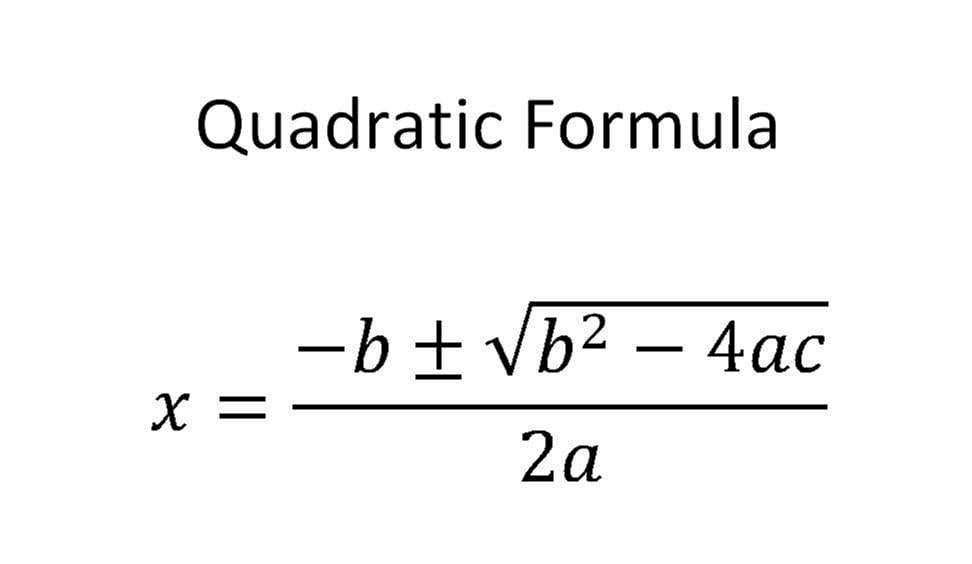
Omelela Dlova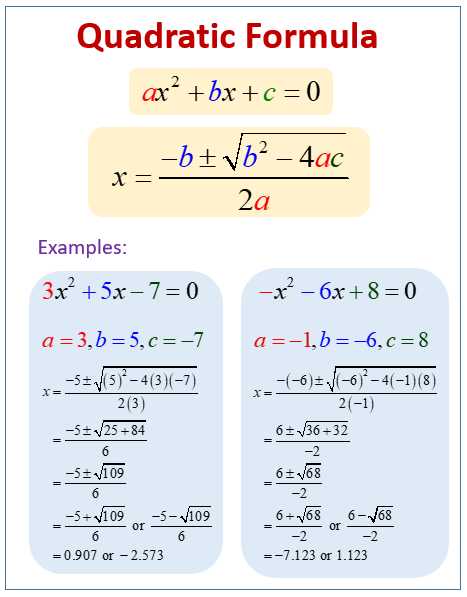
Ahlume Hluma thank you